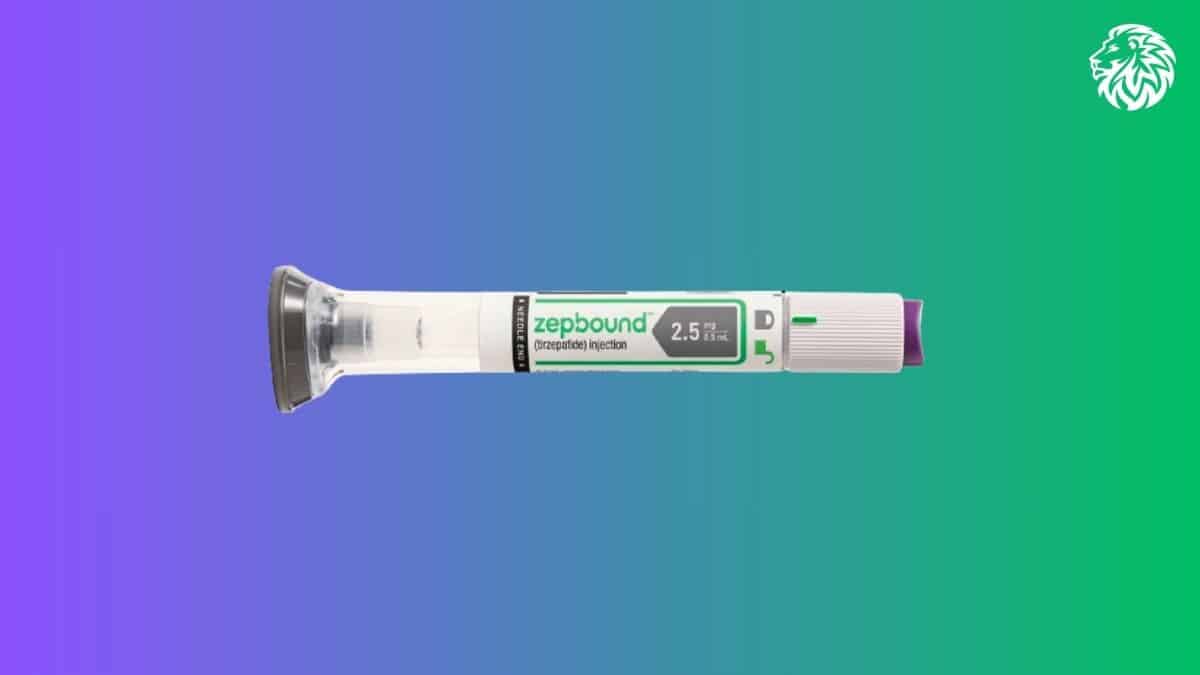
With the rising focus on managing obesity and its related health complications, the approval of Zepbound has generated substantial interest among healthcare providers and individuals looking for effective, sustainable weight-loss options. Zepbound, a medication developed by Eli Lilly, offers new hope as a tool for weight management, especially for adults who face significant challenges due to obesity and weight-related health conditions.
What is Zepbound, and How Does it Work?
Zepbound contains the active ingredient tirzepatide, which operates as a GIP and GLP-1 receptor agonist. This means it mimics two hormones found naturally in the body, GIP (glucose-dependent insulinotropic polypeptide) and GLP-1 (glucagon-like peptide-1), that regulate hunger, digestion, and blood sugar levels. By binding to the GIP and GLP-1 receptors, Zepbound has multiple effects: it slows down the passage of food through the digestive system, prolonging the feeling of fullness, and encourages the pancreas to produce more insulin, effectively managing blood glucose levels.
Zepbound’s mechanism is closely related to other medications designed for blood sugar control and weight management but differs in that it targets both GIP and GLP-1 receptors simultaneously. This dual action enhances its efficacy in appetite suppression, enabling users to consume fewer calories and achieve significant weight reduction.
Clinical Impact and Weight Loss Results
Zepbound is administered once weekly through a subcutaneous injection and is designed for individuals with obesity (BMI of 30 or greater) or those who are overweight (BMI of 27 or greater) with weight-related medical issues, such as high blood pressure or type 2 diabetes. Studies, particularly the SURMOUNT-1 clinical trial, highlight its effectiveness: participants who used Zepbound at a 15 mg dose weekly lost an average of 52 pounds (around 23.6 kg) over 72 weeks, equating to a 21% weight reduction. This impressive outcome underscores Zepbound’s potential to redefine weight management protocols.
In comparison, other commonly used weight-loss medications such as Wegovy also yield significant results, but Zepbound’s dual-hormone targeting has shown greater efficacy in some clinical settings. The option to start on a lower dose, typically at 2.5 mg, and gradually increase the dosage provides flexibility and helps mitigate potential side effects.
Common Side Effects and Considerations
Like any medication, Zepbound has associated side effects, and patients are encouraged to work closely with their healthcare providers to manage them. Common side effects include:
- Gastrointestinal issues such as nausea, vomiting, diarrhea, and constipation.
- Abdominal pain and reflux.
- Fatigue and hair loss in some cases.
- Injection site reactions, including swelling or discomfort.
While these side effects are typically manageable and lessen over time, some individuals may experience more severe reactions. Rare but serious side effects can include acute pancreatitis, gallbladder disease, and in patients with type 2 diabetes, the potential for worsening diabetic retinopathy (eye damage due to diabetes). Additionally, there are rare reports of allergic reactions or kidney issues in some patients, necessitating immediate medical attention.
Of particular importance are the thyroid-related warnings associated with Zepbound. Although thyroid tumors have not been observed in human trials, tirzepatide led to thyroid tumors in animal studies. Thus, individuals with a personal or family history of medullary thyroid carcinoma (MTC) or those diagnosed with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) are advised against using this medication. Regular follow-ups and laboratory tests can help in monitoring the body’s response to Zepbound, minimizing risks associated with long-term use.
Zepbound vs. Other Weight Loss Medications
Zepbound’s active ingredient, tirzepatide, is also found in Mounjaro, a medication specifically approved for managing type 2 diabetes. Though both medications are similar in composition and delivery method (weekly injections), Mounjaro is not FDA-approved for weight loss. By contrast, Zepbound is explicitly designed for weight management in non-diabetic patients or those with weight-related complications.
Comparatively, Wegovy (semaglutide) and Ozempic also belong to the GLP-1 receptor agonist class, but they do not activate GIP receptors. This differentiation positions Zepbound as a potentially more effective solution for weight management, especially for individuals seeking a significant percentage of body weight reduction.
Dosage and Administration Guidelines
The standard starting dose for Zepbound is 2.5 mg injected weekly. After four weeks, the dosage is usually increased to 5 mg weekly. Further dose increases can be considered after at least four weeks on the current dose, with 15 mg as the maximum recommended weekly dose.
Zepbound is administered under the skin in the thigh, abdomen, or upper arm, and users are encouraged to rotate injection sites each week to reduce the risk of local skin irritation. The flexibility of adjusting the dosing day, as long as it remains at least three days apart, can make adhering to a weekly regimen easier for patients.
Safety Precautions: Who Should Avoid Zepbound?
Certain groups may need to avoid or take extra precautions when considering Zepbound:
- Pregnant or breastfeeding individuals: Zepbound’s effects on an unborn child or infants are not fully known, making it essential for these individuals to discuss alternative options with their healthcare providers.
- Individuals with gastrointestinal or kidney issues: Zepbound slows gastric emptying, which could worsen conditions like gastroparesis (delayed stomach emptying). Those with kidney complications should also proceed with caution.
- Patients using birth control pills: Since Zepbound may reduce the effectiveness of oral contraceptives, it’s advisable to consider alternative birth control methods, especially during dosage adjustments.
Cost, Accessibility, and Insurance Coverage
The cost of Zepbound may vary based on insurance coverage and pharmacy options. Savings programs are available for those with commercial insurance, potentially reducing costs to $25 per month for eligible individuals. However, those covered by government programs, such as Medicare, may not qualify for these discounts. For self-pay patients, Zepbound’s single-dose vials offer a more affordable option.
Patients should verify their insurance coverage and explore options through LillyDirect, Eli Lilly’s direct-to-patient service, which can offer access to single-dose vials at competitive prices. This approach ensures that Zepbound remains accessible to a broader demographic, particularly those without extensive insurance support.
Related: Wegovy, Saxenda, Ozempic, Mounjaro
Conclusion: Embracing Zepbound as a Tool for Lasting Weight Management
Zepbound stands as a promising new entry in the realm of weight management medications, combining GIP and GLP-1 receptor activation for more comprehensive appetite and glucose regulation. By complementing traditional lifestyle changes, such as diet and exercise, with Zepbound, many individuals can experience meaningful, sustained weight loss. While some users may encounter side effects, careful dose adjustments and routine medical monitoring can significantly enhance safety and effectiveness.
As with any medication, consulting a healthcare provider is crucial to developing a weight management plan that addresses both personal health needs and potential risks. For those struggling with obesity or weight-related health conditions, Zepbound could be a transformative solution, offering renewed hope in achieving long-term health and well-being.
Frequently Asked Questions About Zepbound
How does Zepbound work for weight loss?
Zepbound targets GIP and GLP-1 receptors to reduce appetite and slow digestion, making you feel fuller longer, aiding in weight loss.
Who is eligible to use Zepbound?
Zepbound is approved for adults with a BMI of 30 or higher or those with a BMI of 27 or higher with weight-related medical issues like type 2 diabetes, hypertension, or sleep apnea.
How much weight can I expect to lose with Zepbound?
In clinical studies, patients lost an average of 20-21% of their body weight, which is about 35-52 pounds over 72 weeks, depending on dosage and adherence.
What are the common side effects of Zepbound?
The most frequent side effects include nausea, vomiting, diarrhea, constipation, abdominal pain, and fatigue, which may lessen over time.
Are there serious risks associated with Zepbound?
Some patients may face risks such as acute pancreatitis, gallbladder disease, kidney issues, or allergic reactions, and those with thyroid tumors or specific endocrine disorders should avoid it.
How is Zepbound administered?
Zepbound is a weekly injection given under the skin in areas like the abdomen, thigh, or upper arm, with rotating injection sites to reduce irritation.
Can pregnant or breastfeeding women use Zepbound?
Due to unknown effects on pregnancy and breastfeeding, Zepbound is not recommended for use during pregnancy or while breastfeeding without medical advice.
Does Zepbound interfere with birth control?
Yes, it may reduce the effectiveness of birth control pills, so additional contraceptive methods are advised when starting or adjusting the dose.
Can I take Zepbound with other weight-loss medications?
Zepbound’s safety when combined with other weight-loss drugs has not been established; consult your doctor for guidance on combining treatments.
Is Zepbound covered by insurance?
Coverage varies by insurance plan, but discounts and savings cards may reduce the cost to as low as $25 per month for those with commercial insurance.
References
- Eli Lilly and Company. (2024, October 24). Lilly releases Zepbound® (tirzepatide) single-dose vials, expanding supply and access for adults living with obesity. Lilly Investor Relations. https://investor.lilly.com/news-releases/news-release-details/lilly-releases-zepboundr-tirzepatide-single-dose-vials-expanding
- Eli Lilly and Company. (2024). Zepbound (tirzepatide) injection for subcutaneous use: Full prescribing information. https://pi.lilly.com/us/zepbound-uspi.pdf
- U.S. Food and Drug Administration. (2023, October 17). FDA approves new medication for chronic weight management. https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management
- Jastreboff, A. M., Aronne, L. J., Ahmad, N. N., Wharton, S., Connery, L., Alves, B., … & Kelly, M. (2023). Tirzepatide once weekly for the treatment of obesity. The New England Journal of Medicine, 387(11), 979–988. https://doi.org/10.1056/NEJMoa2206038
- PR Newswire. (2023, October 17). FDA approves Lilly’s Zepbound™ (tirzepatide) for chronic weight management, a powerful new option for the treatment of obesity or overweight with weight-related medical problems. https://www.prnewswire.com/news-releases/fda-approves-lillys-zepbound-tirzepatide-for-chronic-weight-management-a-powerful-new-option-for-the-treatment-of-obesity-or-overweight-with-weight-related-medical-problems-301982041.html
- Lovelace, B. (2024, October 30). Why sales of Eli Lilly’s Zepbound and Mounjaro fell short. CNBC. https://www.cnbc.com/2024/10/30/why-sales-of-eli-lillys-zepbound-and-mounjaro-fell-short.html
- Hoffman, J. (2024, October 3). Mounjaro and Zepbound: Shortage and compounded tirzepatide. The New York Times. https://www.nytimes.com/2024/10/03/well/mounjaro-zepbound-shortage-compounded-tirzepatide.html
- WebMD. (2024). Zepbound (subcutaneous) medication details. https://www.webmd.com/drugs/2/drug-187794/zepbound-subcutaneous/details